Every Day Matters
Delivering on our vision is a team effort and together we’re undaunted by the challenge. Using our expertise in synthetic biology, we’re developing a novel pipeline of TIL therapies in oncology, with our first product due to advance into patients in 2022 and a focused pipeline of therapeutics in preclinical development.
Through our collaborations with BMS and Vertex, we’re applying our cytoDRiVE® platform technology to enable discovery of novel therapies with the potential to expand our impact and reach a wider range of patients.
Our Culture
We know that patients are waiting, and we’re fiercely committed to delivering transformative therapies as fast as we responsibly can. We act with integrity and challenge ourselves and each other to bring our best every day.
We’re constantly questioning our assumptions, expanding our knowledge, generating and testing new ideas and approaches. Our curiosity drives our innovation.
We don’t believe in silos or hierarchies. We share ideas, strive and learn together, trust each other, and appreciate the value that every member of our team contributes.
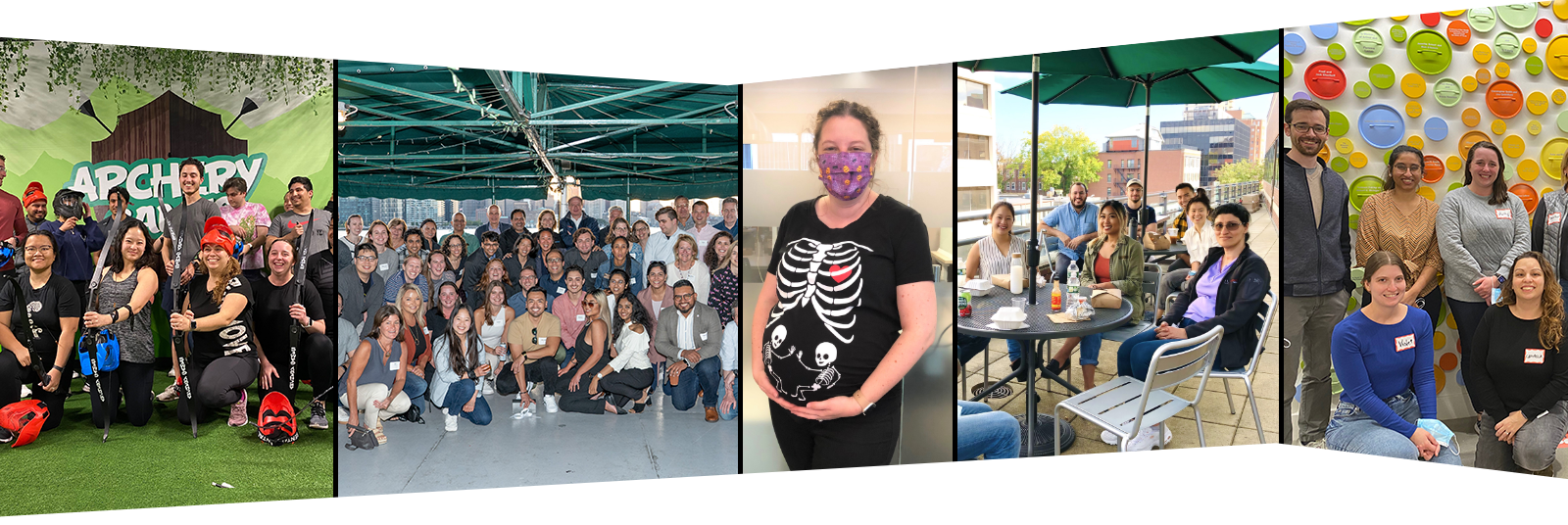
We believe that healthy relationships and high performing teams develop when we can be our whole selves. We make time to connect, have fun, and get to know each other’s stories and interests.
Like the diversity of obsidian sources, our team brings varied backgrounds, identities, and experiences. Our collective knowledge, intellect, and energy is our greatest source of strength.
Testimonials
“One of the main reasons I joined Obsidian still rings true – there is a lot of room to take on important responsibilities and learn new things that will help us build our program and see it succeed.”
–Lauren McLaughlin, Associate Director, Clinical Operations
“There is such camaraderie and cross-functional collaboration between the Analytical and Process Development teams which is fantastic! And within Analytical Development everyone is helpful, supportive, and critical of each other’s work in a caring way that helps us to learn and drive better results.”
–Amanda Logan, Senior Associate Scientist, Analytical Development
“We have a real platform that can make a real difference in people’s lives, allowing us to dose in immuno-oncology what we couldn’t otherwise because it was too toxic.”
–Dhruv Sethi, Senior Director, Discovery & DRD Technologies
“It’s not just an opportunity but an expectation that we each have skills to contribute, and take initiative to expand, grow, and have a real impact.”
–Mara Inniss, Director, Gene Editing Regulation
“We’re very patient centric and focused on how our work can translate to patient response, which is very inspiring to me. We’re at the forefront of TIL therapy, the challenges make it interesting and there is synergy between research and tech ops when we work hand in hand.”
–Zheng Ao, Principal Scientist, Cell Therapy
“The #1 reason I joined was the science. I thought it was an interesting take on cell therapy, I liked the evidence that TILs work, and I can see a benefit for the patients.”
–Nicole Hsu, VP, Regulatory Affairs

Benefits
Career Growth
Diverse experiences, cross-functional collaboration, mentorship, training programs, and tuition reimbursement
Career matrices (a tool for planning future development), and professional development goal setting
Financial
We’re committed to gender pay equity
Employee stock options, 401K, and health savings accounts provide long-term value
Commuter benefit: reimbursement for public transport or parking
Well Being
Generous coverage and low out of pocket costs for health and dental care (including a generous annual Health Savings Account contribution)
Perks to keep us well including gym, massage therapy, and meditation reimbursements, and team competitions like “Move it March”
Time Off
We work hard to advance our mission, and are encouraged to relax and refresh under our flexible time off program
Six (6) weeks of fully paid bonding leave for parents to welcome a new child (birth, adoption, or foster care), in addition to fully paid medical maternity leave
Perks
We connect over company-sponsored lunches on our deck overlooking Mass Ave near the heart of Harvard Square, walks or rides on the Minuteman Bikepath right next to our Bedford facility, and through company sponsored social events